Expanded Capacity for Vial & Syringe Packaging at Keystone Folding Box Co.
Press release from the issuing company
Amid increased demand from biologics and vaccines sectors, Company invests in additional die cutting and gluing machinery, expanding production capacity and allowing for expedited turnaround.
Newark, N.J. – Keystone Folding Box Co., a designer and manufacturer of paperboard packaging solutions, has incorporated new die cutting and gluing equipment into its production facility. The infrastructure investment increases the company’s production capacity of vial and syringe packaging by 25%, and accommodates shorter lead-times.
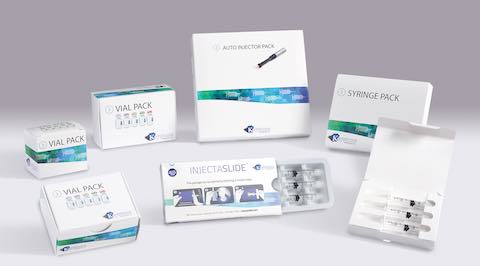
Keystone offers a comprehensive line of secondary packaging systems for injectable pharmaceutical products. Available in a variety of tailored features and in both all-paperboard and plastic-hybrid formats, solutions are available for prefilled syringes, autoinjector pens, vials and ampoules, as well as combinations of these drug delivery formats.
In particular, developments in the biologics and vaccines markets have led to heightened demand for custom packaging solutions for vial and prefilled syringe products. During the past six months, Keystone has seen significantly increased requests in these sectors, producing packaging solutions for pharma customers manufacturing monoclonal antibodies, vaccines, recombinant hormones/proteins, and cellular- and gene-based biologics, among other niches.
According to a survey from Zion Market Research, the global vaccine market is expected to exceed $59 billion in 2020 – a figure that has nearly doubled in just six years. In the United States alone, the vaccines market is anticipated to reach $18 billion. The biologics market is growing at an even more exponential rate; according to current market reports, the global biologics market was valued at over $250 million in 2018, and is expected to exceed $625 million by 2026.
To meet the building demand for packaging solutions in these sectors, Keystone’s secondary packaging collection meets a range of needs through options such as tamper evidence and child-resistance via reclosable locking mechanisms – all while ensuring parenteral product protection throughout the pharmaceutical supply chain. Solutions include:
- A line of Injectable Packs is suitable for prefilled syringes or autoinjector pens, and can be designed to include ancillary items like sterile wipes
- The InjectaSlide package incorporates a thermoformed tray that slides inside a carton and into a locked position; a lock release button must be pressed to unlock and slide the tray forward for easy access to each medicine dose.
- The Vial Pack line of cartons includes designs for packaging a single vial or ampoule, or as many pharmaceutical products as a program requires.
“With injectable products made of glass or rigid plastic, protection is paramount.” said Ward Smith, Director of Marketing & Business Development at Keystone Folding Box Co.. “Secondary packaging must be designed and produced with the right materials to protect prefilled syringes, autoinjectors, vials and ampoules as they travel through the supply chain.”
- KYOCERA NIXKA INKJET SYSTEMS (KNIS) INTRODUCES BELHARRA, THE NEW WAVE OF PHOTO PRINTERS
- New RISO Printing Unit Offers Easy Integration for Package Printing
- March 2024 Inkjet Installation Roundup
- Inkjet Integrator Profiles: Integrity Industrial Inkjet
- Revisiting the Samba printhead
- 2024 Inkjet Shopping Guide for Folding Carton Presses
- The Future of AI In Packaging
- Inkjet Integrator Profiles: DJM

WhatTheyThink is the official show daily media partner of drupa 2024. More info about drupa programs
© 2024 WhatTheyThink. All Rights Reserved.









Discussion
Join the discussion Sign In or Become a Member, doing so is simple and free