Aptar CSP Technologies to Host Webinar on "Rethinking Oral Solid Dose Packaging"
Press release from the issuing company
Rethinking Oral Solid Dose Packaging Extending Shelf Life, Improving Stability and Expediting Speed to Market Webinar Hosted by Aptar CSP Technologies
New solution to old challenges: Packaging-based solutions for oral solid dose stability challenges associated with moisture, oxygen and volatile compounds
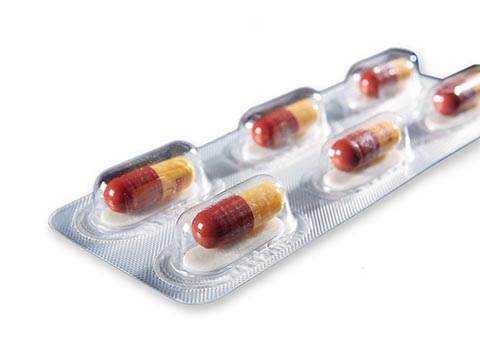
Auburn, Ala. – Aptar CSP Technologies – a leader in active packaging solutions that ensure product protection, enhance brand recognition and improve use experiences – is presenting its Activ-Blister™ Solutions for the protection of moisture and oxygen-sensitive tablets and capsules, alongside FreeThink Technologies and its ASAPprime® technology for accelerated shelf-life determination. The combination is a “right the first time” approach to stability challenges and blister package design that virtually eliminates protracted testing and costly reformulations. The webinar, titled Rethinking Oral Solid Dose Packaging, will be conducted on March 28 at 11:00 a.m. EST.
Activ-Blister™ solutions control the internal atmosphere of individual blister cavities, allowing for improved product performance and enhanced shelf-life. Using proprietary three phase polymer technology, engineered materials can absorb customized amounts of water vapor, oxygen, and/or volatile compounds, and can be produced in shapes and sizes to accommodate any tablet and capsule size. This innovative technology can be applied via heat-staking, without the use of adhesives, and added to existing packaging lines.
Working together with FreeThink Technologies, a leader in accelerated shelf-life determination, and utilizing their ASAPprime® technology, a drug product’s moisture and oxygen sensitivity is determined using highly accelerated stability studies open to specified environmental conditions – such as temperature, relative humidity and oxygen level. With this analysis, modeling creates a theoretical blister design with Activ-Blister™ solutions to achieve desired shelf life.
Once the optimized blister and sorbent are determined, laboratory, clinical and stability study packaged product can be manufactured. This total process from Aptar CSP Technologies is called Xcelerate and provides expedited end-to-end expertise for packaging of sensitive oral solid doses, from R&D to commercial launch.
The combination of Activ-Blister™ solutions and ASAPprime® technology is in response to the increased complexity of matching moisture- and oxygen-sensitive oral dose products to ideal packaging solutions, as well as to the ever-growing demand to expedite time to market. Tablets and capsules are today’s most popular drug delivery forms, and stability issues are only likely to increase with the development of more potent API’s, larger molecules and modified release profiles. Innovative and evolving dosage forms, such as chewable and disintegrating tablets, also face heightened stability challenges and problems of shelf life. Adding to all of this, global distribution demands in zone 4a/4b (hot/humid) are also increasing.
“Activ-Blister™ solutions and the Xcelerate complete packaging service offer improved stability, extend shelf-life and expedite time to market for sensitive oral solid dose products,” said Craig Voellmicke, Vice President, Business Development, Aptar CSP Technologies. “Together, they provide a valuable combination of effective drug protection and ease of adoption.”
- Questions to ask about inkjet for corrugated packaging
- Can Chinese OEMs challenge Western manufacturers?
- The #1 Question When Selling Inkjet
- Integrator perspective on Konica Minolta printheads
- Surfing the Waves of Inkjet
- Kyocera Nixka talks inkjet integration trends
- B2B Customer Tours
- Keeping Inkjet Tickled Pink
© 2024 WhatTheyThink. All Rights Reserved.














