Schreiner MediPharm Develops New Security Label Providing Tamper-Evidence for Vials
Press release from the issuing company
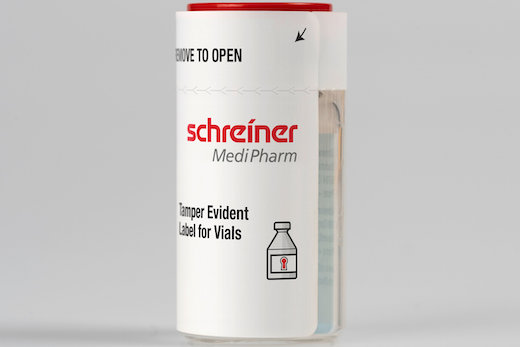
Due to its clear first-opening indication, the new security label from Schreiner MediPharm protects the integrity of vials and helps avoid their illegal reuse.
Solution offers clear first-opening indication for small pharmaceutical containers.
Blauvelt, NY – Schreiner MediPharm, a Germany-based global provider of innovative functional label solutions for the healthcare industry, has introduced a tamper protection label with clear and irreversible first-opening indication for vials.
Drug counterfeiters frequently fill original pharmaceutical containers with ineffective or harmful substitutes. In many cases, container tampering cannot be detected, and in the worst cases a falsified medicine could even cause fatal consequences for patients. Developed specifically for vials, Schreiner MediPharm’s new solution can help prevent illegal reuse of original containers and protect their integrity.
The new security label wraps around the vial up to the level of the cap. To open the vial, a label-integrated tear strip must be peeled off, and cannot be resealed unnoticed. A warning message clearly indicating that the vial has been opened also emerges. As an optional, additional layer of protection, this first-opening indication can be boosted by a void effect feature in which previously invisible lettering or symbols separate from an indicator field.
For enhanced security, verification features for proof of authenticity also can be incorporated into the label. Furthermore, special features are available such as a detachable documentation label, a transparent inspection window for checking vial content, a light protection function for sensitive substances, or integrated NFC/RFID chips for digital applications.
Schreiner MediPharm’s new tamper-evident label is especially well-suited for small vials, and can be custom-designed to meet various applications. It can be processed in the normal labeling workflow in pharmaceutical production while preserving the product’s branding and design. Pharmaceutical manufacturers benefit from a solution that is precisely adapted to their requirements, easily fits their production processes, and helps protect their supply chain. For healthcare professionals, the label is not only easy to use but, above all, indicates at first glance whether the product is an originally sealed medication. As a result, patients can be protected against the potential administration of ineffective or harmful medications.

WhatTheyThink is the official show daily media partner of drupa 2024. More info about drupa programs
© 2024 WhatTheyThink. All Rights Reserved.









Discussion
Join the discussion Sign In or Become a Member, doing so is simple and free